RJPS Vol No: 15 Issue No: 3 eISSN: pISSN:2249-2208
Dear Authors,
We invite you to watch this comprehensive video guide on the process of submitting your article online. This video will provide you with step-by-step instructions to ensure a smooth and successful submission.
Thank you for your attention and cooperation.
1Neha Shivathaya, Assistant Professor, Department of Pharmaceutics, Rani Chennamma College of Pharmacy, Belagavi, Karnataka, India.
2Department of Pharmaceutics, Sree Siddaganga College of Pharmacy, Tumkur, Karnataka, India
3Department of Pharmaceutics, Sree Siddaganga College of Pharmacy, Tumkur, Karnataka, India
4Department of Pharmaceutics, Rani Chennamma College of Pharmacy, Belagavi, Karnataka, India
5Department of Pharmaceutics, Rani Chennamma College of Pharmacy, Belagavi, Karnataka, India
*Corresponding Author:
Neha Shivathaya, Assistant Professor, Department of Pharmaceutics, Rani Chennamma College of Pharmacy, Belagavi, Karnataka, India., Email: nehashivathaya21@yahoo.com
Abstract
Background: Psoriasis is a skin disorder characterized by hyperproliferation of skin cells and an inflammatory response involving neutrophils. Wrightia tinctoria has traditionally been used in treating psoriasis, and various studies have supported its effectiveness. The study investigated the phytochemical contents of Wrightia tinctoria leaves and determined the existence of rutin, a bioactive substance thought to have therapeutic effects on psoriasis.
Aim: The study aimed to assess the therapeutic potential of Wrightia tinctoria plant leaf extract by assessing its phytochemical composition and determining the marker molecule rutin using Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) analysis.
Methods: Fresh Wrightia tinctoria leaves were collected and analyzed using qualitative and quantitative microscopy. The phytochemical profile was determined using an aqueous extract of dried leaf powder. A sensitive RP-HPLC method was devised to detect the rutin concentration in aqueous leaf extract.
Results: The phytochemical analysis revealed the presence of alkaloids, carbohydrates, flavonoids, sterols, and tannins. A good linearity was observed in the chromatographic data. The retention time of rutin was found to be 3.122 min at 256 nm. The concentration of rutin in the extract was 8.62 µg/mL. The reported value of chromatographic parameter was identified by comparing with standard solution having retention time of 3.198 min at 10 µg/mL.
Conclusion: The pharmacognostical evaluation of Wrightia tinctoria leaves provides useful information for standardization protocols. The short retention time in the RP-HPLC method indicates fast and efficient chromatographic separation, enabling quicker and more reliable analysis of rutin.
Keywords
Downloads
-
1FullTextPDF
Article
Introduction
Psoriasis is a hyperproliferative neutrophilic skin disorder. It is the most common long-lasting chronic autoimmune inflammatory skin disease.1 According to the World Health Organization (WHO), it distresses 1.5-5% of the population in developing countries and 0.9-11.4% worldwide.2 According to the degree of inflammation, 85% to 90% of psoriatic individuals have vulgaris, or plaque.3 Psoriasis is a T-cell mediated disease that arises from faulty signal processing in the immune system, wherein keratinocytes multiply and reach the skin surface rapidly from the basal layer within 6-8 days, unlike normal skin, and gather on the epidermis instead of shedding, resulting in non-evident lesions.4
Wrightia tinctoria is a well-known medicinal plant, and its leaves have traditionally been used to cure psoriasis. It belongs to the family Apocynaceae. This herb is effective in the treatment of dandruff and other scalp and skin diseases. Phytochemical and pharmacological studies on various parts of the plant revealed anti-ulcer, anti-cancer, anti-dandruff, anti-anxiety, antipsoriatic, antifungal, antibacterial, antiviral, anti-inflammatory, anti-diabetic, analgesic, hepatoprotective, anthelmintic, and wound healing properties. Preliminary phytochemical analysis of Wrightia tinctoria methanolic extract revealed the presence of alkaloids and flavonoids. Similarly, its leaves contain carbohydrates, steroids, phenols, saponins, flavonoids, tannins, and protein.5 Wrightia tinctoria is widely used in the treatment of psoriasis. Various studies have documented the topical and oral efficacy of the plant in curing psoriasis.6
Considering the above facts, the study aimed to comprehensively identify the phytochemical constituents present in Wrightia tinctoria leaves, with a specific focus on quantifying rutin using a reverse phase-high performance liquid chromatography (RP-HPLC) method, a potential marker for psoriasis based on its anti-inflammatory and antioxidant properties. The study provides valuable insight into the therapeutic potential of Wrightia tinctoria leaves and may serve as a foundation for further investigation into the plant's effectiveness in treating psoriasis.
Materials and Methods
Fresh leaves of Wrightia tinctoria were obtained from medicinal nurseries in Kelamangala, Hosur, Tamil Nadu, India, in August 2023. The leaf was authenticated by Harsha Hegde, Scientist-E at ICMR- NITM, Belagavi. The herbarium specimen of this has been deposited in their herbaria with accession number. The chemicals and reagents used were of pharmaceutical or laboratory reagent grade.
Macroscopic (Organoleptic) Evaluation
Wrightia tinctoria fresh leaves were examined under a microscope for organoleptic characteristics such as colour, odour, taste, shape, margin, apex, base and surface.7
Microscopic Evaluation (Qualitative Microscopy)
Wrightia tinctoria fresh leaves were sectioned for transverse section (T.S.) analysis.8
Quantitative Microscopy9-11
Determination of vein islet number
A small square section of the laminar area was cleaned in chloral hydrates, stained and mounted on a glass slide. A light camera was installed, and the paper was divided into 1 mm² squares using a bench-top micrometer and a 16 mm lens. After the preparation was cleared, the stage micrometer was replaced, and the veins were raced in four continuous squares measuring either 2 mm X 2 mm or 1 mm X 4 mm. During counting, each vein-islet was numbered in the table. Each numbered zone comprised a complete series of veins, and cells intersected by the upper margins or obscured within a square (or rectangle) were excluded, whereas those overlapping the other two margins were included. Ten measurements of vein-islet number were recorded.
Determination of stomatal number and stomatal index
It is the percentage of stomata to total epidermal cells, with each stoma counted as one cell. Stomatal index (I) = S/S + E X 100, where S represents the number of stomata per unit area, and E represents the number of epidermal cells in the same unit area. The technique for determining stomatal number was observed under high power (45X). Epidermal cells and stomata were counted. The stomatal index was computed from these data using the formula provided above.
Palisade ratio determination
A leaf slice was cleared in chloral hydrate and examined under a microscope. The camera lucida and drawing board were set up, and a 4 mm objective was used to trace the contours of four epidermal cells. After focusing on the palisade layer, the outlines of the palisade cells beneath the epidermal walls were traced. Palisade cells below the four epidermal cells were counted. Five sets of four epidermal cells from different areas of the leaf were analyzed, and the average number of palisade cells beneath the epidermal cells was calculated as the palisade ratio.
Powder Microscopy12-15
The dried leaf was powdered and treated with standard chemical reagents to identify the plant's diagnostic features. The resulting powder was examined under a microscope, where various chemical treatments helped reveal characteristic structures and cellular components. Photographs of these features were captured through photomicrography.
Physico-Chemical Standardization
Determination of foreign organic matter
An accurately weighed 100 g of air-dried coarse drug was spread in a thin layer. The sample was examined with the naked eye or under a 6x lens, and any foreign organic matter was manually separated and weighed.
Loss on drying
A tarred spray can was filled with 10 g of carefully weighed, coarsely powdered drug. The plate was dried at 105 °C for 5 hours and then weighed. Drying and weighing were repeated at hourly intervals until the difference between two consecutive weighings was no greater than 0.25%. The loss on drying was computed as a percentage of the powdered sample.
Determination of total ash
The ash content of the crude drug, representing inorganic salts naturally present, adhering to it, or those intentionally added as adulterants, was determined by the residue remaining after incineration. Three grams of air-dried, coarsely powdered drug were placed in a pre-weighed silica crucible and incinerated at a temperature not exceeding 450 °C until all carbon was removed. After cooling, the residue was weighed, and the percentage of ash was calculated based on the weight of the air-dried drug.
Determination of acid insoluble ash
The ash from the previous method was mixed with 25 cc of 2 M hydrochloric acid and heated for five minutes in a water bath. The insoluble residue was filtered using ashless filter paper, rinsed with hot water, dried, and ignited for 15 minutes at 450 °C. After cooling in a desiccator, the residue was weighed. The percentage of acid-insoluble ash was estimated using the weight of the air-dried medication.
Preparation of Aqueous Extract of Wrightia tinctoria Leaves
Healthy Wrightia tinctoria leaves were harvested and dried in the shade at room temperature. The dried material was ground in a mixer, sieved, and stored in a tightly closed container in a dry place. A hot aqueous extract was prepared using distilled water (60 g of leaf powder in 600 mL of DW; 10% W/V) at a constant temperature of 60 °C. The extract was then filtered using Whatman filter paper No. 1.16,17
Preliminary Phytochemical Profile of Wrightia tinctoria Plant Leaf18-22
The aqueous extract of Wrightia tinctoria leaf was subjected to qualitative chemical analysis. Various chemical assays were used to identify flavonoids, phenolic compounds, alkaloids, glycosides, carbohydrates, carotenoids, proteins, tannins, amino acids, and sterols.
Identification and Characterization of the Marker Compound Rutin by Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
Technique Rutin, a polyphenolic hydrophobic molecule with antioxidant and anti-inflammatory properties, has been investigated as a potential treatment for psoriasis. Researchers studied the antipsoriatic effects of rutin at a dose of 100 mg/mL using a mouse tail model and HaCaT cells.23
The marker compound was identified by RP-HPLC and compared with standard rutin utilizing a C18 column. The mobile phase consisted of acetonitrile and 0.05% OPA (orthophosphoric acid) (1:1 ratio), with a flow rate of 0.7 mL/min, and detection was carried out at 256 nm.24,25
Preparation of standard and sample solution
Five milligrams of standard biomarker rutin was dissolved in 10 mL of methanol to achieve a concentration of 500 µg/mL, labeled as stock solution I. Aliquots of 0.1, 0.2, 0.3, 0.4, and 0.5 mL of the stock solution were diluted to 10 mL with the mobile phase to obtain concentrations of 5, 10, 15, 20, and 25 µg/mL, respectively. The sample extract solution was prepared by dissolving 10 mg of extract in 10 mL of methanol to obtain a final concentration of 1000 µg/mL.
RP-HPLC instrumentation
The pump unit used was a G1310A IsoPump (HP-1100 reciprocating pump) with a maximum pressure of 400 bar and a pressure display accuracy of 5%. The discharge rate was 0.001-5 mL/min. Up to four mobile phases could be employed for elution with a mixing ratio range of 0-100%.26,27
Chromatographic conditions
Chromatographic separation was performed using a Younglin (S.K) gradient system equipped with a UV detector (UV 730D) and a pump (SP930 D). The system was controlled by Autochro-3000 software. Separation was achieved on a Phenomenex C18 column (4.6 x 250 mm, 5 µm particle size). The mobile phase consisted of a 50:50 mixture of acetonitrile and 0.05% OPA (orthophosphoric acid), while the stationary phase was RP C18 (Thermo). The flow rate was set at 0.7 mL/ min and the sample volume was 20 µL. The detection was performed at a wavelength of 256 nm. Rutin was identified by comparing the retention time of the sample with the standard. All experiments were conducted under identical conditions at ambient temperature. The quantitative estimation of rutin was performed by comparing the RP-HPLC peak areas of the standard and the sample.28
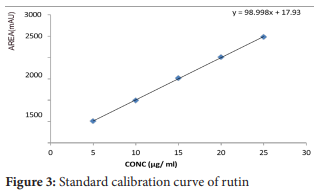
Preparation of standard calibration curve
Five different concentration levels of mixed reference solutions were analyzed, with rutin concentrations of 5-25 μg/mL. A calibration curve was constructed using the formula: Y = a x + b, where the Y-axis represents the peak area (mAU), and the X-axis corresponds to the concentration (μg/mL) injected into the RP-HPLC column for each standard.29
Results
The plant was authenticated by Harsha Hegde, Scientist-E at ICMR-NITM, Belagavi. The herbarium specimen was deposited in their herbaria with accession number RMRC-1802.
Macroscopical Studies
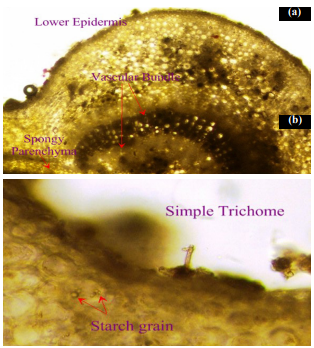
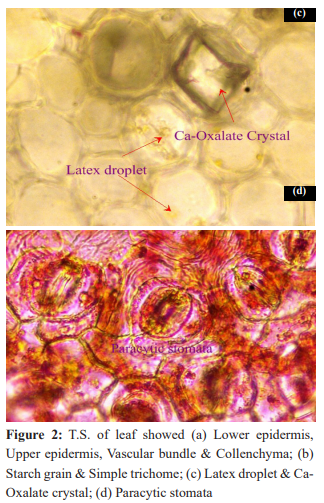
The transverse section (T.S.) of the Wrightia tinctoria leaf revealed a dorsiventral structure. The upper and lower epidermis consisted of a single layer of tangentially elongated, compact cells, both bearing uniseriate covering trichomes. The mesophyll was differentiated into palisade and spongy parenchyma, with scattered rosette crystals. The palisade tissue extended to the midrib region, beneath which spongy parenchyma was observed. The midrib contained vascular bundles arranged in a ring, with xylem centrally located and phloem at the periphery, encircled by pericyclic fibers. A collenchymatous strip was present below the upper epidermis of the midrib, while the remaining portion consisted of a loosely arranged parenchyma. Stomata were confined to the lower epidermis and identified as paracytic type.
Quantitative Microscopy
Fresh leaves showed the following parameters within Indian Pharmacopoeia (IP) range: vein islet number (4-6), stomatal index (40-47), palisade ratio (5-11), and vein termination number (8-10).
Powder Microscopy
The crude powder exhibited a coarse texture, green colour, no distinct odour, and an astringent taste.
Physicochemical Constants
Analysis of the crude leaf powder showed:
- Loss on drying: 7.39 ± 0.4% w/w
- Total ash: 8.18 ± 0.06% w/w
- Water-soluble ash: 6.4 ± 0.20% w/w
- Acid-insoluble ash: 0.040 ± 0.023% w/w
All values were within the specified IP limits.
Phytochemical Screening
The aqueous extract tested positive for carbohydrates, alkaloids, flavonoids, sterols, and tannins.
HPLC Analysis
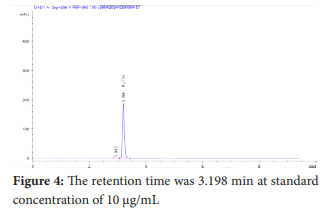
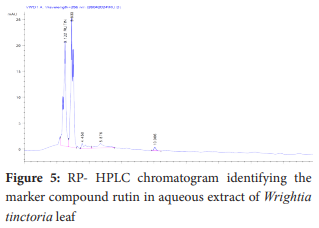
The standard compound exhibited a retention time of 3.198 min at a concentration of 10 µg/mL. The sample extract showed a retention time of 3.122 min at 256 nm at a concentration of 8.62 µg/mL. The low retention time indicated rapid chromatographic separation.
Discussion
The macroscopical and microscopical characteristics observed in Wrightia tinctoria leaves, including the dorsiventral structure, paracytic stomata, and presence of rosette crystals, align with the diagnostic features reported for this species. These characteristics are valuable for authentication and differentiation from possible adulterants.
Quantitative microscopy parameters, such as vein islet number, stomatal index, palisade ratio, and vein termination number, were within the standard IP range, thereby confirming the identity and quality of the plant material.
The physicochemical constants, including ash values and moisture content, were within acceptable pharmacopoeial limits, indicating that the crude drug is of good quality and free from excessive inorganic matter.
The phytochemical screening suggested the presence of major bioactive constituents like alkaloids, flavonoids, and tannins, supporting the ethnopharmacological use of the plant. The presence of sterols further adds to its therapeutic potential.
The HPLC analysis demonstrated a short retention time, which reflects efficient chromatographic separation and supports the reliability of the phytochemical profiling.
Overall, the findings validate the pharmacognostic standards of Wrightia tinctoria leaves and provide baseline data for its quality control, authentication, and potential pharmaceutical applications.
Conclusion
The quantitative analysis was carried out using a calibration curve created with standard rutin solutions at various concentrations. The correlation coefficient exceeded 0.9997, indicating excellent linearity. This method was then applied to determine the rutin content in the aqueous extract of Wrightia tinctoria leaf powder. The chromatogram showed a distinct rutin peak with a retention time of 3.122 minutes and a concentration of 8.62 µg/mL. To confirm the chromatographic parameters, the sample's retention time was compared to the standard solution, which had a retention time of 3.198 minutes at a concentration of 10 µg/mL. The results demonstrate that the developed RP-HPLC method for quantifying rutin in the antipsoriatic herbal extract is effective, offering a broad range of linearity, a simple mobile phase, UV detection, a short retention time, and no requirement for an internal standard. Rutin, a major flavonoid in the plant leaf extract, can be qualitatively and quantitatively estimated using this method, making it suitable for analysis of both single and compound herbal formulations.
Conflict of interests
The authors state that there is no conflict of interest with the publishing of this paper.
Supporting File
References
- Sondhi S, Singh N, Goyal K, et al. Development of topical herbal gel of berberine hydrochloride for the treatment of psoriasis. Research Journal of Pharmaceutical Dosage Forms and Technology 2021;13(1):12-8.
- Fereig SA, El-Zaafarany GM, Arafa MG, et al. Tackling the various classes of nano-therapeutics employed in topical therapy of psoriasis. Drug Deliv 2020;27(1):662-680.
- Wadher K, Dabre S, Gaidhane A, et al. Evaluation of antipsoriatic activity of gel containing Pongamia pinnata extract on Imiquimod-induced psoriasis. Clin Phytosci 2021;7(20):1-6.
- Pradhan M, Alexander A, Singh MR, et al. Understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed Pharmacother 2018;107:447-63.
- Sangeetha S, Hari A, Pattam S, et al. An updated review on Wrightia tinctoria (Roxb). R Br. J Pharm Res Int 2021;33(56A):234-244.
- Sundarrajan S, Lulu S, Arumugam M, et al. Deciphering the mechanism of action of Wrightia tinctoria for psoriasis based on systems pharmacology approach. J Altern Complement Med 2017;23(11):866-878.
- Patil NV, Bhosale AV, Ubale MB. An overview of pharmacologically and phytochemical significant plant Wrightia tinctoria. International Journal of Pharmacy Pharmaceutical Research 2019;15(1):336-343.
- Dixit A, Jain AK, Tiwari P, et al. A phytopharmacological review on an important medicinal plant - Wrightia tinctoria. Current Research in Pharmaceutical Sciences. 2014;4(3):70-76.
- Patil SG, Wagh AS, Pawara RC, et al. Standard tools for evaluation of herbal drugs: An overview. Pharma Innovation 2013;2(9):60-65.
- Mahadevan N, Moorthy K, Perumal P, et al. Pharmacognosy of leaves of Wrightia tinctoria R. Br. Ancient Science of Life 1998;18(1):78.
- Kumar D, Kumar K, Kumar S, et al. Pharmacognostic evaluation of leaf and root bark of Holoptelea integrifolia Roxb. Asian Pacific Journal of Tropical Biomedicine 2012;2(3):169-75.
- Devi SL, Divakar MC. Pharmacognostical Evaluation on the leaves of Wrightia tinctoria (Roxb) R. Br. Hygeia 2012;4:104-11.
- ridhar S, Kamalakannan P, Elamathi R, et al. Studies on antimicrobial activity, physio-chemical and phytochemical analysis of Wrightia tinctoria R. Br. IJPRD 2011;3(8):139-44.
- Khan Z, Ansari I, Kanase V. A pharmacognosy study of Wrightia tinctoria plant. World J Pharm Res 2017;6:385-91.
- Devi PS, Satyanarayana B, Naidu MT. Phytochemical screening for secondary metabolites in Boswellia serrata Roxb. and Wrightia tinctoria (Roxb.) R. Br. Notulae Scientia Biologicae 2014;6(4):474-7.
- Sharmila R, Hariprasanth S. Phytochemical profiling and in silico docking studies of Wrightia tinctoria against psoriasis. World Journal of Pharmaceutical Research 2018;7(3):649-663.
- Bharani M, Karpagam T, Varalakshmi B, et al. Synthesis and characterization of silver nano particles from Wrightia tinctoria. Int J Appl Biol Pharm Technol 2012;3(1):58-63.
- Tare HL, Gore MS, Deore SR, et al. Comparative hemintholytic potential of extracts obtained from Cymbopogon citratus and Wrightia tinctoria leaves. International Journal of Pharma and Biosciences 2011;2(1):321-6.
- Maddila S, Hemalatha KP. Phytochemical screening and in vitro antimicrobial properties of crude leaf extracts of Wrightia tinctoria R. Br. International Journal of Current Microbiology and Applied Sciences 2017;6(1):707-20.
- Meenu C, Manokari L. Analysis of phytochemical constituents and antibacterial activity of Wrightia tinctoria: traditional medicinal plant of India for application on wound dressing materials. Indian Journal of Traditional Knowledge 2022;21(1):48-54.
- Trease GE, Evans WC. Pharmacognosy. 12th ed. London: Cassell and Collier Macmillian Publishers Ltd.; 1983. p. 734,1985.
- Kokate CK. Practical pharmacognosy. 2nd ed. Pune: Niraliprakashan; 1989.
- Nowak-Perlak M, Szpadel K, Jabłońska I, et al. Promising strategies in plant-derived treatments of psoriasis-update of in vitro, in vivo, and clinical trials studies. Molecules 2022;27(3):591.
- Ashok PK, Saini B. RP-HPLC analysis and isolation of rutin from stem bark of Ginkgo biloba L. J Pharmacogn Phytochem 2013;2(4):68-71.
- Deineka V, Grigor’ev A, Staroverov V. RP-HPLC analysis of flavonoids: determining rutin in plant extracts. Pharmaceutical Chemistry Journal 2004;38(9):487-489.
- Musthaba SM, Athar MT, YT K, et al. Fast analysis and validation of rutin in anti-psoriatic ayurvedic formulation by RP- HPLC. Journal of Liquid Chromatography & Related Technologies 2011;34(6):446-55.
- Doshi GM, Une HD. Quantification of quercetin and rutin from Benincasa hispida seeds and Carissa congesta roots by high-performance thin layer chromatography and high-performance liquid chromatography. Pharmacognosy Res 2016;8(1):37-4.
- Liu D, Mei Q, Wan X, et al. Determination of rutin and isoquercetin contents in Hibisci mutabilis folium in different collection periods by RP- HPLC. Journal of Chromatographic Science 2015;53(10):1680-4.
- Naveen P, Lingaraju HB, Prasad KS. Simultaneous determination of rutin, isoquercetin, and quercetin flavonoids in Nelumbo nucifera by high-performance liquid chromatography method. International Journal of Pharmaceutical Investigation 2017;7(2):94.