RJPS Vol No: 15 Issue No: 4 eISSN: pISSN:2249-2208
Dear Authors,
We invite you to watch this comprehensive video guide on the process of submitting your article online. This video will provide you with step-by-step instructions to ensure a smooth and successful submission.
Thank you for your attention and cooperation.
1Madhuri H R, Assistant Professor, Department of Pharmaceutics, GM Institute of Pharmaceutical Science and Research, GMU Campus, Davangere, Karnataka, India.
2Department of Pharmaceutics, GM Institute of Pharmaceutical Sciences and Research, Davangere, Karnataka, India
3Department of Pharmaceutics, GM Institute of Pharmaceutical Sciences and Research, Davangere, Karnataka, India
4Department of Pharmacology, GM Institute of Pharmaceutical Sciences and Research, Davangere, Karnataka, India
*Corresponding Author:
Madhuri H R, Assistant Professor, Department of Pharmaceutics, GM Institute of Pharmaceutical Science and Research, GMU Campus, Davangere, Karnataka, India., Email: madhu.rayabagi@gmail.com
Abstract
Background: Bioactive compounds derived from medicinal plants have gained prominence in the pharmaceutical industry due to their therapeutic potential and role in enhancing health and disease resistance.
Aims/Objectives: The present study aims to identify the bioactive phytochemicals present in the ethanolic leaf extract of cranberry hibiscus (Hibiscus acetosella) using Gas Chromatography-Mass Spectrometry (GC-MS) and to explore their potential pharmacological properties
Methods: Ethanolic extracts of cranberry hibiscus leaves were subjected to GC-MS analysis to detect and characterize the phytochemical constituents. Identification was based on retention time, molecular weight, and mass spectral data, which were matched against the NIST (2008) GC-MS library.
Results: A total of 27 bioactive compounds were identified from the extract. Major constituents included ethane, 1,1-diethoxy; 1-propanol, 2-methyl; 1-propene, 3-fluoro; acetaldehyde; carbamic acid monoammonium salt; 3-aminobenzhydrazide; and cyclohexane,1-ethenyl-1-methyl-2,4-bis(1-methylethenyl). These compounds are associated with a wide range of pharmacological activities, including antimicrobial, antioxidant, anti-inflammatory, antidiabetic, antifungal, antibacterial, vasodilatory, and potential anticancer effects.
Conclusion: The GC-MS analysis confirms that cranberry hibiscus leaf extract is rich in therapeutically valuable phytochemicals. These findings suggest that it could serve as a promising source for the development of crude drugs and novel therapeutic agents in modern medicine.
Keywords
Downloads
-
1FullTextPDF
Article
Introduction
Cranberry hibiscus, an individual from the Malvaceae family, is an amphidiploid plant local to Africa and is typically consumed as a green vegetable. In traditional Western and Central African medicine, decoction drinks have been prepared from leaf shoot concentrates to harness their anti-anemic and antipyretic properties.1 Cranberry hibiscus leaf decoctions are traditionally consumed in Uganda as a blood-purifying tonic and for the treatment of anemia. Infusions prepared from the leaves are also used as a post-fever tonic. In Nigeria, the plant locallyand known as “Akese”-is used to treat dysentery, regulate menstrual disorders, and manage post-partum conditions.2 Despite the various restorative uses attributed to this plant, pharmacognostic and phytochemical data on Hibiscus acetosella remain limited. In contrast, other Hibiscus species, such as H. sabdariffa and H. rosasinensis, have been extensively studied and reported to possess antioxidant, antimicrobial, and antihypertensive properties.3 However, comparative studies focusing on H. acetosella are scarce, underscoring a significant gap in understanding its therapeutic potential. In recent years, Gas Chromatography-Mass Spectrometry (GCMS) has emerged as a vital analytical tool for profiling secondary metabolites in plants. GC-MS offers high sensitivity and accuracy in identifying and quantifying volatile and semi-volatile bioactive compounds, providing detailed fragmentation patterns that facilitate compound identification and structural elucidation. Despite the limited studies on the pharmacological properties of this plant, the present study aims to establish the GC-MS profile of the ethanolic leaf extract of Hibiscus acetosella and evaluate its pharmacological potential.4,5
Materials and Methods
The plant specimens for the proposed study were collected in September and October in and around Kyatanahalli village, Davanagere (Dist), Karnataka, India. Dr. Aruna Charantimath, Head of the Department of Botany, GM Academy First Grade College, Davanagere, Karnataka, India, authenticated the leaf parts of the cranberry hibiscus. A voucher specimen was stored in our laboratory.
The dried leaves were ground into a coarse powder using an electric blender and sieved through an ASTM No. 40 mesh (425 μm) to ensure uniform particle size. The sieved powder was stored in an airtight container at room temperature until further analysis.
Leaf extract preparation: Approximately 50 grams of dried, coarsely powdered leaves were extracted with 500 mL of ethanol using a Soxhlet apparatus, followed by GC-MS.
GC-MS Analysis: The analysis was conducted using a Clarus 680 gas chromatograph (GC) system from Perkin Elmer, which was equipped with a fused silica column containing Elite-5MS (5% biphenyl and 95% dimethyl-polysiloxane, with dimensions of 30 m × 0.25 mm ID × 250 μm film thickness). Helium served as the carrier gas, maintaining a constant flow rate of 1 mL/min to ensure adequate separation of the compounds. The injector temperature was kept at 260°C throughout the chromatographic run, and a 1 μL sample of the extract was injected into the system. The oven temperature program started at 60°C for 2 minutes and then increased at a rate of 10°C per minute until it reached 300°C, where it was held for an additional 6 minutes. The mass spectrometer operated under specific conditions: the transfer line temperature was set to 240°C, the ion source temperature was also at 240°C, and electron impact ionization was performed at 70 eV. The scan time was configured to 0.2 seconds, with a scan interval of 0.1 seconds, allowing it to detect fragments within a mass range of 40 to 600 Da.6
Identification of components: Bioactive compounds were identified by interpreting the mass spectra obtained from GC-MS analysis using a GC-MS NIST (2008) library. The mass spectra of unknown compounds were compared with those of known compounds stored in these libraries. The names, molecular formulas, and molecular weights of the identified compounds were tabulated.
Results
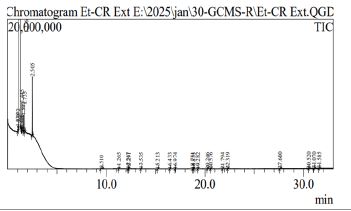
The GC-MS chromatogram of the ethanolic leaf extract of cranberry hibiscus revealed 27 peaks, indicating the presence of 27 distinct components (shown in Figure 1).
The molecular weight, molecular structure, height (%), area (%), retention time (RT), and peaks of the active chemicals are summarized in Table 1.
The ethane, 1,1-diethoxy-1-propanol, 2-methyl-1-propene, and 3-fluoro showed the maximum percentage. Acetaldehyde, carbamic acid, monoammonium salt, 3- aminobenzhydrazide, 1-propene, 2-fluoro showed moderate percentage and cyclohexane, 1-ethenyl-1 methyl-2, 4-bis (1-methylethenyl), A-norcholestan-3- one, 5-ethenyl-(5 beta), 1,3-dioxolane, 2-heptyl showed the minimum percentage.
The compounds identified through GC-MS exhibit various pharmacological activities, as outlined in Table 2, based on literature reports. The detected compounds were found to possess a range of biological properties, including antioxidants, antibacterial, anti-inflammatory, antimicrobial, and anticancer activities.
Discussion
The GC-MS analysis confirmed the chemical diversity of cranberry hibiscus leaves, revealing compounds with a broad spectrum of pharmacological activities. The high abundance of ethane, 1,1-diethoxy, and 1-propanol, 2-methyl, known for their anti-inflammatory and antibacterial effects, respectively, suggests potential antimicrobial and anti-inflammatory applications of the extract. The presence of 1-propene, 3-fluoro, an established antibacterial agent, further reinforces the antimicrobial potential of the extract against pathogenic microorganisms.
Several compounds, such as methylsulfidiole, 3-hexonone, 2,5-dimethylpentanal, and 9,12,15-octadecatrienal, are reported to exhibit antioxidant activity, which aligns with the traditional use of cranberry hibiscus in preventing oxidative stress-related disorders. Antioxidants are known to neutralize free radicals, thereby contributing to disease prevention and improved health outcomes. The presence of 3-aminobenhydrazide and A-norcholestan-3-one is particularly due to their reported anticancer properties. These compounds may contribute to chemopreventive effects, offering potential in cancer therapy. Additionally, ethyl acetate, hexadecanal, and diethyl phthalate demonstrated antimicrobial properties, suggesting that the extract could serve as a natural source of bioactive agents against bacterial infections.
Overall, the phytochemical profile obtained supports the ethnopharmacological use of cranberry hibiscus. The combination of antioxidant, antimicrobial, anti-inflammatory, and anticancer constituents underscores the plant's potential as a source of therapeutic agents. However, further in vitro and in vivo studies are required to validate these activities and determine the safety and efficacy of the identified compounds.
Conclusion
The present study successfully identified 27 bioactive compounds in the ethanolic leaf extract of cranberry hibiscus (Hibiscus acetosella) using Gas Chromatography-Mass Spectrometry (GC-MS). The presence of phytoconstituents holds significant pharmacological potential. These compounds are associated with a broad spectrum of biological activities, including antioxidant, antimicrobial, anti-inflammatory, antidiabetic, anti-fungal, vasodilatory, and possible anticancer effects. The findings support the traditional medicinal use of cranberry hibiscus and highlight its promise as a natural source for developing novel therapeutic agents. Further in vitro and in vivo pharmacological evaluations are recommended to validate the therapeutic efficacy and safety of these bioactive constituents. Overall, this study contributes to the growing scientific evidence supporting the medicinal value of herbal plants and encourages the continued exploration of Hibiscus acetosella in pharmaceutical research.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this research paper.
Funding
This research was financially supported by Rajiv Gandhi University of Health Sciences, Karnataka, under the University Research Grant Scheme (Project Code: UG24PHA0692). The authors gratefully acknowledge the funding support provided.
Supporting File
References
1. Ssegawa P, Kasenene JM. Medicinal Plant Diversity and Uses in the Sangobay Area, Southern Uganda. J Ethnopharmacol 2007;113(4):521-40.
2. Polska wersja. Hibiscus acetosella - Cranberry Hibiscus. [internet] 2016 [cited 2025 April 27]. Avail-able from: Herbs from Distant Lands: Hibiscus acetosella - Cranberry Hibiscus
3. Alharbi AE, AlHussaini AM, Alshami I. A Comprehensive Review of the Antimicrobial Effects of Hibiscus Species. Cureus 2024;16(11):e73062.
4. Banakar P, Jayaraj M. GC-MS analysis of bioactive compounds from ethanolic leaf extract of Waltheria indica Linn. and their pharmacological activities. Int J Pharm Sci Res 2018;9(5):2005-10.
5. Gomathi D, Kalaiselvi M. GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) L. J Food Sci Technol 2013;50(12):2438-44.
6. Anusiya G, Bharathi S. Extraction and molecular characterization of biological compounds from water hyacinth. J Med Plants Stud 2020;8(5):14-9.
7. Al-Rahbi BAA. Effectiveness of endophytic and rhizospheric bacteria from Moringa spp. in controlling Pythium aphanidermatum damping-off of cabbage. Novel Biocontrol Tools Res Plant Prot 2023;12(3):668-72.
8. Sarc L, Wraber B. Ethanol and acetaldehyde disturb TNF-α and IL-6 production in cultured astrocytes. Sage Publication 2011;30(9):741-8.
9. Yan HL, Cao SC. Effects of methylsulfonylmethane on growth performance, immunity, antioxidant capacity, and meat quality in Pekin ducks. Poult Sci 2020;99(2):1069-74.
10. Wang X, Wang Y, Ren Q, Li L. Fluorinated organic compounds: Their synthesis and potential antibacte-rial activity. J Fluor Chem 2018;205:62-75.
11. Kumar S, Sharma P. Antimicrobial potential of ethyl acetate extract from fungal symbionts. J Appl Pharm Sci 2020;10(4):75-82.
12. García M, López R, Martín J. Inhibitory effects of isobutanol on bacterial growth: A potential antibacterial agent. Appl Microbiol Biotechnol 2019;103(2):789-98.
13. Zhao H, Liu J, Wang Z. Structural modifications and biological activities of ethoxy derivatives in pharmaceutical applications. Eur J Med Chem 2020;193:112-21.
14. Patel H, Shah K, Desai P. Synthesis and biological evaluation of hydrazone derivatives as potential antimicrobial and anti-inflammatory agents. J Med Chem Res 2018;27(3):245-60.
15. Kumar A, Verma S. Biological activities of hydrazone derivatives: A review on their antioxidant and anti-inflammatory potential. Curr Pharm Des 2019;25(4):567-80.
16. Smith J, Brown R. Role of 3-aminobenzhydrazide as a PARP inhibitor in cancer therapy. Int J Oncol Res 2020;15(2):112-25.
17. Kuçuk HB. Synthesis and biological activity of new 1,3-dioxolanes as potential antibacterial and anti-fungal compounds. Molecules 2011;16(8):6806-15.
18. Rahim M, Azad A. A review on spiro cyclopro-panes: Their significance in medicinal chemistry and antimicrobial properties. J Iran Chem Soc 2017;14(12):2311-25.
19. Silva RA, Almeida PR. Essential oils with siloxane compounds: A review on their antimicrobial and anti-cancer properties. Front Pharmacol 2020;11:1254.
20. Premjanu N, Jayanthy C. Antimicrobial activity of diethyl phthalate: An in-silico approach. Asian J Pharm Clin Res 2014;7(4):165-8.
21. Mondal A, Maity TK, Bishayee A. Analgesic and anti-inflammatory activities of quercetin-3-methoxy-4′-glucosyl-7-glucoside isolated from Indian medicinal plant Melothria heterophylla. Medicines. 2019;6(2):59.
22. 2,5-Dimethyl-3-hexanol. [Internet]. [cited 2025 April 27]. Available from: https://www.benchchem.com BenchChem 2023.
23. Yang M. Synthesis and fungicidal activities of (Z/E)-3,7-dimethyl-2,6-octadienamide and its 6,7-epoxy analogues. Molecules 2015;20(12):21023-36.
24. Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogen-ic bacteria. Pharmaceuticals (Basel) 2013;6(12):1451-74.
25. Mucha P, Skoczyńska A, Małecka M, Hikisz P, Budzisz E. Overview of antioxidant and anti-inflammatory activities of selected plant compounds and their metal ion complexes. Molecules 2021;26(16):4886.
26. Nossaman VE. Nitrates and nitrites in the treatment of ischemic cardiac disease. Cardiol Rev 2010;18(4):190-7.
27. Jiang Y, Kim B, Zhang X, R et al. Composition and antioxidant activities of volatile organic compounds from Coreopsis rosea Nutt. Molecules 2020;25(14):3170.
28. Ramesh S, Subramanian M. Chemical composition and antimicrobial properties of Pittosporum dasycaulon Mig. Essential oil. J Essent Oil Res 2018;30(5):345-52.
29. Zhang L, Wang Y, Li J, Chen H. Volatile composition and antioxidant activity of Brassica oleracea extracts. Food Sci Nutr 2023;11(3):4321-32.
30. Adepoju G, Bello O. Chemical composition and antimicrobial activity of essential oils from Jasminum sambac and Plumeria alba flowers. Trop J Nat Prod Res 2022;6(2):125-34.
31. Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med 2006;6:39.
32. Choudhary E, Bithel N, Sharma T, Saini P, Rajput M. GC–MS characterization of Eupatorium odoratum (L.) leaves essential oil and evaluation of in vitro antimicrobial and antioxidant activity. J Pure Appl Microbiol 2023;17(4):8645-54.
33. Smith J, Patel R, Kumar A. Anticancer effects of norcholestane derivatives in vitro and in vivo: Mechanistic insights and therapeutic implications. J Med Chem 2019;62(7):3456-65.
34. Johnson M, Lee Y. Evaluation of antioxidant and anti-inflammatory activities of modified steroid compounds. J Ethnopharmacol 2020;250:112-20